




Check out this awesome and smooth color on last year Nexletol paint scheme! Chris Buescher
.
.
.
#NASCAR #nexletol #chrisbuescher #rfkracing #fordmustang




Good news for Neuland Labs.
USFDA has approved Esperion's new label expansions for NEXLETOL (bempedoic acid) & NEXLIZET (bempedoic acid & ezetimibe) for cardiovascular risk reduction
More than 70 million patients to now be eligible for NEXLETOL and NEXLIZET in US
#neulandlabs

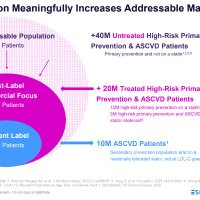
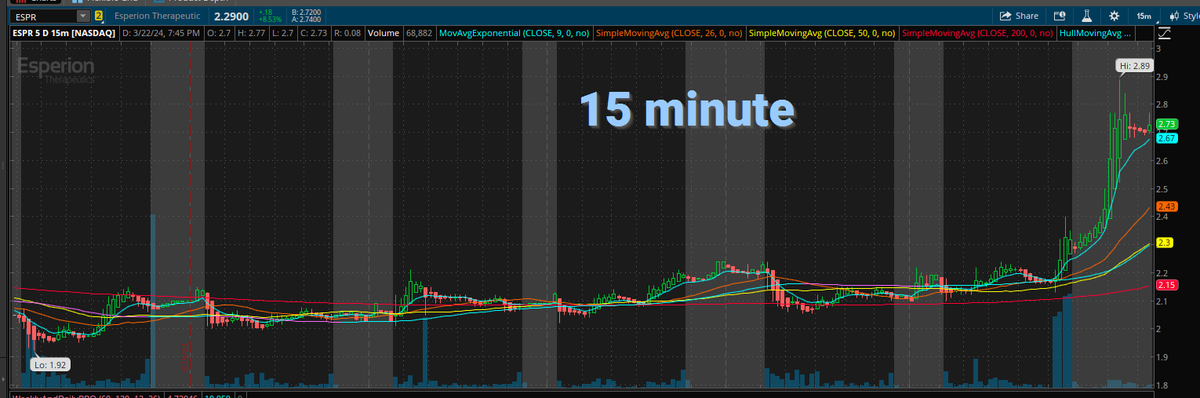
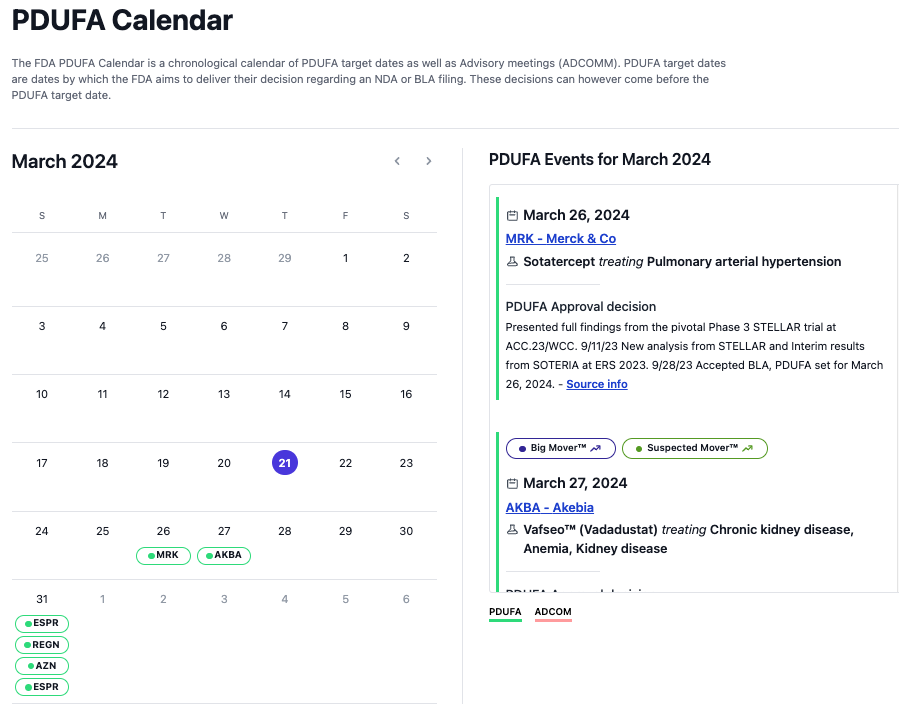
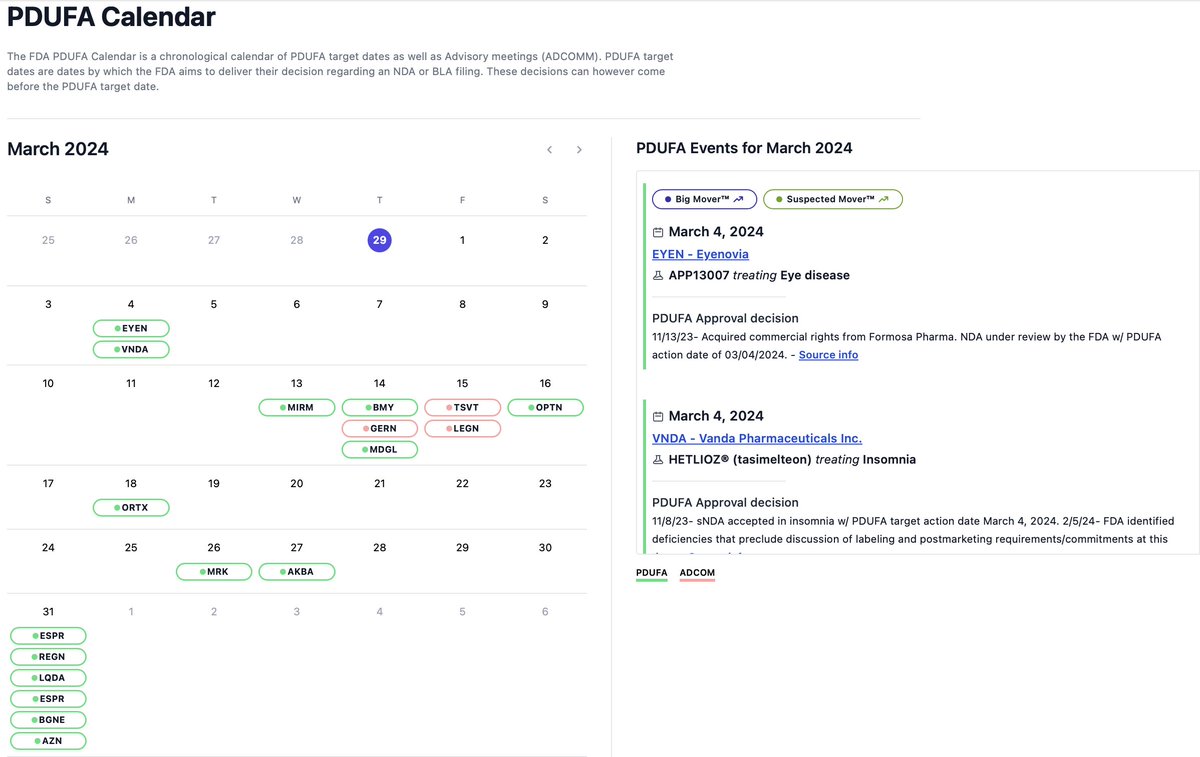
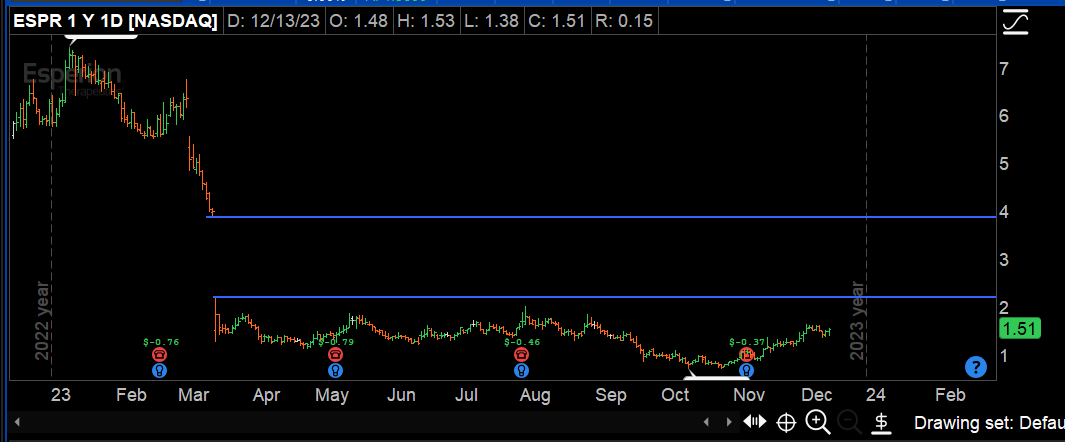
wallstreetbets $ESPR $2.38is the most undervalued #biotech wallstreetbets They Two FDA Approved non- #statin medications #nexlizet & #nexletol had label expansion 2 months ago & now is available to 70M+ Americans. Yesterday great label news for partner DSE. 2M scripts expected by 2027 =$50+


Is your practice taking care of patients facing #statin -intolerance? #primarycare
In a long-term study, bempedoic acid (Nexletol) significantly reduced the risk of heart attacks, strokes, and other cardiovascular events (39.9 months). #BempedoicAcid #HeartHealth



#EsperionTherapeuticsInc U.S. FDA Approves Broad New Labels for NEXLETOL® and NEXLIZET® to Prevent Heart Attacks and Cardiovascular Procedures in Both Primary and Secondary Prevention Patients, Regardless of Statin Use globenewswire.com/news-release/2…





Advancing an Anti-Cholesterol Drug Compound at the Height of the Pandemic. #BempedoicAcid #Nexletol #Nexlizet Esperion Inc. neulandlabs.com/blog/2023/04/1…


.Esperion Inc. is committed to developing innovative medicines to improve outcomes for patients with or at risk for cardiovascular and cardiometabolic diseases.
To mark the FDA’s approval of expanded indications for NEXLETOL and NEXLIZET, #NasdaqListed $ESPR is ringing the