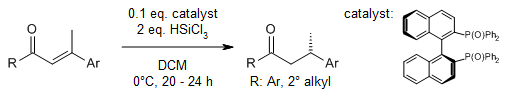
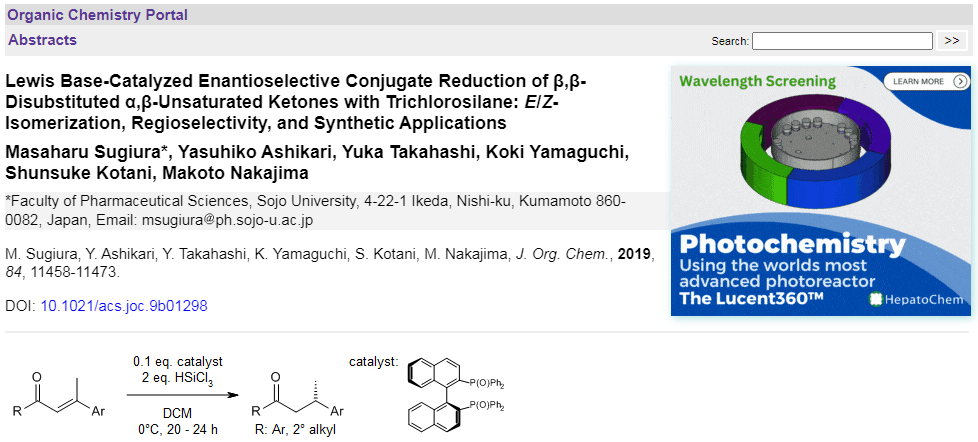
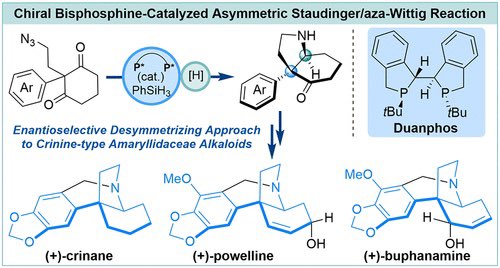
Chiral Bisphosphine-Catalyzed Asymmetric Staudinger/aza-Wittig Reaction: An Enantioselective Desymmetrizing Approach to Crinine-Type Amaryllidaceae Alkaloids
J. Am. Chem. Soc. #Chemistry #Chemed #Science #TechnologyNews #news #technology #AcademicTwitter
pubs.acs.org/doi/10.1021/ja…

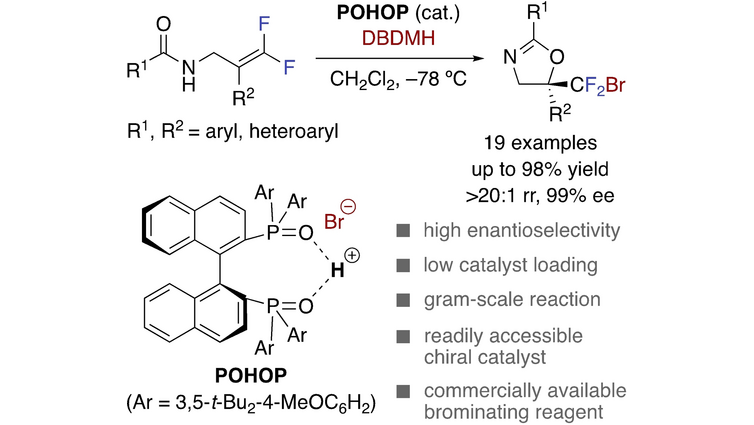
Enantioselective Synthesis of Bromodifluoromethyl-containing Oxazolines by Concerted Lewis/Bronsted Base #Catalysis with Chiral Bisphosphine Oxide. Yoshitaka Hamashima et al. of University of Shizuoka onlinelibrary.wiley.com/doi/10.1002/as…


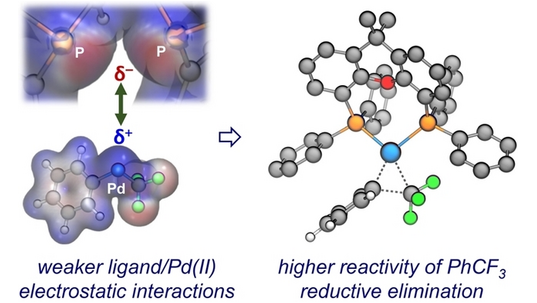
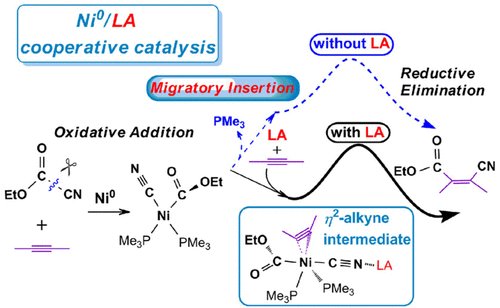
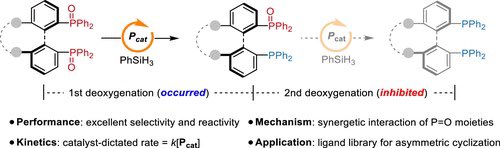
Phosphonium-Catalyzed Monoreduction of Bisphosphine Dioxides: Origin of Selectivity and Synthetic Applications
J. Am. Chem. Soc. #Chemistry #Chemed #Science #TechnologyNews #news #technology #AcademicTwitter #AcademicChatter
pubs.acs.org/doi/10.1021/ja…


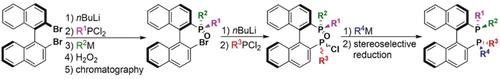
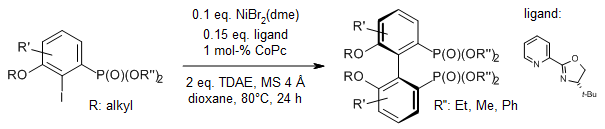
Raphael Kim from DAWatsonLab Univ. of Delaware presented his graduate work ACS Organic Division 2021 GRS! Very nice talk Raphael on “Development of a Nickel-Catalyzed Atropselective Ullmann Coupling en route to Chiral Biaryl Bisphosphine Ligands” #GRS2021 #2021GRS



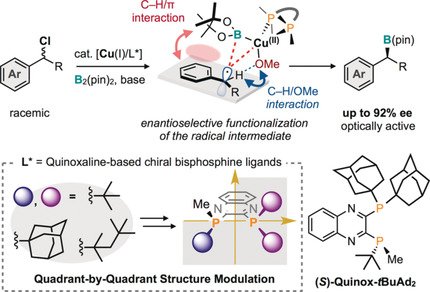
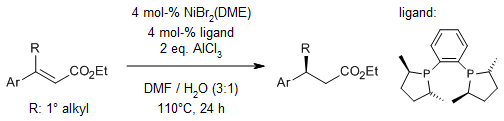
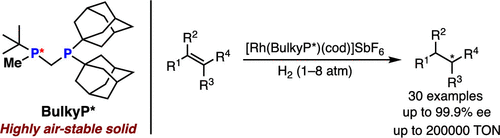
Nippon Chemical team reports on the Rh-catalyzed asymmetric hydrogenation of alkenes with TON of up to 200 000 with a new, stable bulky three-hindered quadrant bisphosphine ligand, ee up to 99%; 30 examples J Org Chem/Org Lett .
pubs.acs.org/doi/10.1021/ac…






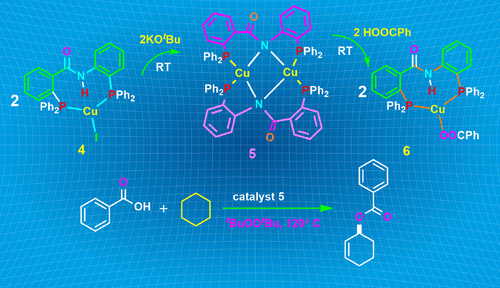
Cu(I) Complexes of Amide Functionalized Bisphosphine: Proximity Enhanced Metal–Ligand Cooperativity and Its Catalytic Advantage in C(sp3)–H Bond Activation of Unactivated Cycloalkanes pubs.acs.org/doi/10.1021/ac… Ramakrishnan, Balakrishna, & co-workers Inorganic Chemistry #copper #catalysis